Factory Anastrozole, Arimidex Anti-estrogen powder available in USA, Canada UK
Product name: China Anastrozole white powder ingredient
Trade name: Arimidex
origin: china supplier
CAS:120511-73-1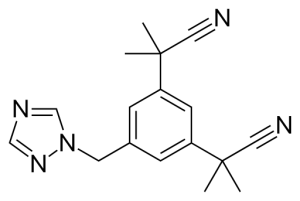
MF: C17H19N5
MW: 293.37
ASSY: 99% Anastrozole
Melting point: 81.2~82.9°C
Appearance: white crystalline powder.
Anastrozole Anti-estrogen powder
 Anastrozole is a drug used to treat breast cancer after surgery. Anastrozole is aromatase inhibitor.Anastrozole inhibits the enzyme aromatase,Anastrozole binds reversibly to the aromatase enzyme through competitive inhition .Anastrozole can be used in man and woman.
Anastrozole is a drug used to treat breast cancer after surgery. Anastrozole is aromatase inhibitor.Anastrozole inhibits the enzyme aromatase,Anastrozole binds reversibly to the aromatase enzyme through competitive inhition .Anastrozole can be used in man and woman.
Anastrozole is a powerful anti-estrogen medication commonly sold under the brand name Arimidex and it is by that name most are familiar with the compound. As an anti-estrogen medication Arimidex belongs to the aromatase inhibitor. Arimidex is not an anabolic steroid; it is not a steroid in any shape or form but is commonly used in conjunction with anabolic steroids to combat estrogenic related side-effects.
Anastrozole Arimidex cycle
In the treatment of breast cancer, Arimidex is almost always dosed at 1mg per day until the cancer subsides. Use may continue for a time at this stage and will often be switched to Nolvadex in a preventative measure once the cancer is in remission.
For the anabolic steroid user, Arimidex doses can vary with 0.5-1mg every other day being the most common. Very few should ever need more than 1mg every other day and many will be more than fine with half that amount. In therapeutic plans such as low testosterone treatment even less may be needed. We can, however, make an exception in Arimidex doses for competitive bodybuilders. Competitive bodybuilders may find a full 1mg every day the last 10-14 days leading up to competition to be useful. This will greatly aid in hardening, but it will be draining to say the least. Of course, at this stage of a competition diet most have very little energy to begin with anyway.

CERTIFICATE OF ANALYSIS
PRODUCT NAME: ANASTROZOLE
BATCH NUMBER: 20170908 MFG. DATE: 2017/09/08
TEST DATE: 2017/09/08 EXP.DATE: 2019/09/07
| ANALYSIS分 | SPECIFICATIONS | RESULTS |
| Assay(on dry basis) | 98%~102%(HPLC) | 99.90% |
| CHEMICAL CONTROL | ||
| Solubiltity | Freely soluble in methanol, ethanol, ethylacetate and chloroform, slightly soluble in water and 0.1M hydrochloric acid solution |
Conforms |
| Indentification | IR Reactive |
Conform with standard IR Conforms |
| Melting point | 82.0 ~ 85.0℃ | 83.1 ~ 83.3℃ |
| Related substances Impurity B Impurity C Impurity D Impurity E Single Unknown Impurity Total Unknown Impurity Total Impurity |
NMT 0.2% NMT 0.1% NMT 0.5% |
0.04% 0.06% 0.28% |
| Residual solvents Dichloromethane Chloroform Ethanol Cyclohexane Ethylacetate |
NMT 0.06% |
ND |
| Residue on ignition | NMT 0.1% | 0.04% |
| PHYSICAL CONTROL | ||
| Description | White or off-white crystalline powder | Conforms |
| Water | NMT 0.3% | 0.12% |
| Heavy metals | NMT 0.001% | <0.001% |
| STORAGE | Cool and dry | |
| SHELF LIFE | 24 months | |
CONCLUSION:THE RESULTS CONFORM USP36 STANDARD.